Human Microbiome Market Definition
Industry Perspective:
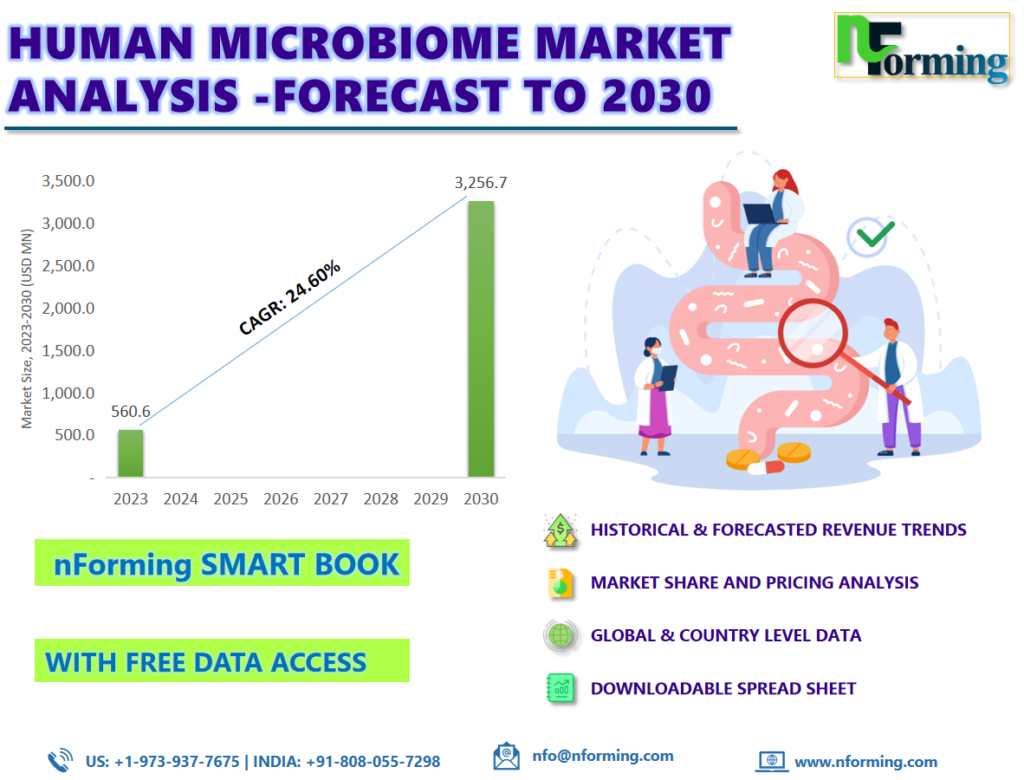
The global human microbiome market size was worth USD 560.57 million in 2022 and is estimated to grow to USD 3,256.65 million by 2030, with a compound annual growth rate (CAGR) of approximately 24.60% over the forecast period. The report analyzes the human microbiome market’s drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the human microbiome market.
Human Microbiome Market: Overview
The group of bacteria that are present in the human body is referred to as the “human microbiome”. The variety of microorganisms found in the human microbiome includes bacteriophages, bacteria, fungi, viruses, and protozoa. Microbiological cells outnumber human cells in human cells by a factor of 10. The weight of the microbiota could reach five pounds. Our microbiome’s bacteria aid in the regulation of our immune system, aid in food digestion, protect us from pathogenic bacteria and produce vitamins like B and K, which are necessary for blood coagulation. In addition, the market is growing as a result of the greater focus on creating treatments for the human microbiome. Its suitability as a therapeutic research target has also been confirmed. A wide range of development opportunities are anticipated to be made available to market players throughout the projection period by the increasing number of partnerships amongst market participants.
nForming Human Microbiome Market Smart Book
The nForming Human Microbiome Market Smart Books offer a comprehensive compilation of data pertaining to historical revenue, user volume, patient data, prices, and other relevant information for each segment. This includes products and services, type of indication, age group, and detailed analysis of service providers with coverage at both regional and country levels. The data spans the past three years and provides projections up until 2030. The smart book also provides extensive information on the leading suppliers and their corresponding market shares in the human microbiome market. The human microbiome y market comprises a range of qualitative factors, including significant growth drivers, opportunities, and challenges.
Human Microbiome Market: Recent Developments
The human microbiome industry is seeing a variety of developments. Among the major development areas are:
Recent Developments
- In July 2023, The US FDA designated Infant Bacterial Therapeutics’ IBP-1016 bacterial product as an orphan medicine, which treats new-borns with gastroschisis.
- In April 2023, Finch Therapeutics and Brigham and Women’s Hospital entered into a clinical trial agreement to assess CP101, a new microbiome treatment, for ulcerative colitis.
Read more opportunities in the human microbiome market in the Smart Book.
Human Microbiome Market Drivers
Recent developments in microbiome-based therapeutics, such as live biotherapeutic products (LBPs) to drive market growth
The market for human microbiome is expanding significantly due to the recent developments in microbiome-based therapeutics, such as live biotherapeutic products (LBPs). The development of recently innovative microbiome-based treatments, such as live biotherapeutic products (LBPs), is anticipated to propel the expansion of the human microbiome market. For example, in Nov. 2022, the U.S. Food and Drug Administration (FDA) approved REBYOTA (faecal microbiota, live-jslm), a novel drug based on microbiota-based live biotherapeutic, which is indicated for the prevention of Clostridium difficile (C. difficile) infection in individuals 18 years of age and older, following antibiotic treatment for recurrent CDI. Ferring B.V. is a global pharmaceutical company.
Human Microbiome Market: Restraints
Negative effects of extensive regulatory laws on the sale of microbiomes hinders market growth.
The commercialization of microbiomes may be negatively impacted by intricate regulatory laws. Navigating regulatory frameworks presents special hurdles for microbiome research due to its dynamic and continuously developing character. Protracted approval procedures, exorbitant expenses, and exacting specifications may impede the prompt creation and introduction of microbiome-driven goods and treatments. Small and medium-sized businesses (SMEs) and startups are disproportionately affected by these barriers, which make it harder for them to compete with more established, larger businesses. Additionally, differing laws in various nations or regions might obstruct access to international markets and hinder commercialization attempts even more. To encourage innovation and guarantee the ethical and effective commercialization of microbiome-based solutions, regulatory bodies and industry stakeholders must work together to streamline and harmonise policies, provide clear guidelines, and stimulate collaboration.
Read all the major drivers and trends of the human microbiome market in the Smart Book.
Human Microbiome Market: Regional Landscape
North America dominated the Human Microbiome market in 2022
Due to a strong foundation of healthcare facilities, rising investments from large corporations in the creation of more advanced technology, a rise in the number of drug development procedures, and an increase in research activities, North America currently holds a dominant position in the market. The market is expanding as a result of rising rates of lifestyle diseases, a focus on human microbiome therapies, and advances in next-generation sequencing and human microbiome technology. However, it is projected that strict regulatory requirements, a lack of experience, and thorough inspection will have a negative impact on market growth in the upcoming years.
Read more competitive developments in the human microbiome Market Smart Book.
Human Microbiome Market Key Companies
Seres Therapeutics, Inc. (US)
Enterome (France)
4D pharma plc (UK)
International Flavors & Fragrances Inc. (US)
OptiBiotix Health Plc (UK)
Ferring Pharmaceuticals (Switzerland)
Synlogic, Inc. (US)
Second Genome, Inc. (US)
Vedanta Biosciences, Inc. (US)
YSOPIA Bioscience (France)
FlightPath Biosciences, Inc. (US)
Finch Therapeutics Group, Inc. (US)
AOBiome Therapeutics (US)
BioGaia (Sweden)
Quantbiome, Inc. (dba Ombre) (US)
Viome Life Sciences, Inc. (US)
BIOHM Health (US)
DayTwo (US)
Atlas Biomed (UK)
Bione Ventures Private Limited (India)
Luxia Scientific (France)
Metabiomics (US)
Sun Genomics (US)
Seed Health (US)
Gnubiotics Sciences (Switzerland).
Human Microbiome Market Segmentation:
The global human microbiome market has been segmented into product, disease, application, and type.
Based on product, drugs, diagnostic tests, probiotics, prebiotics, and other products are segments of the global human microbiome market. The drugs segment dominated the market in 2022. The substantial share of this segment may be mainly ascribed to elements like the rise in financing for drug development and the number of microbiome-based medications that are being developed.
Based on disease, the market is classified into infectious disease, gastrointestinal diseases, endocrine & metabolic disorders, cancer, and other diseases. In 2022, the infectious disease category dominated the global market. Treatment for infectious diseases that specifically target bacteria is now more important than ever due to the rising awareness of the detrimental effects of antibiotic use on natural flora, including disruptions. The major factors driving this segment’s expansion are the rising number of clinical studies for the development of target-specific microbiome-based therapies and the increased prevalence of disorders caused by microbial dysbiosis as a result of antibiotic therapy.
Based on application, the market is classified into therapeutics, and diagnostic. In 2022, the therapeutics category dominated the global market. One important element fueling the expansion of this application field is the rise in R&D spending for drugs based on the microbiome. In addition, advances in technology, a rise in clinical trials in the diagnostics sector, and increasing collaborations between major industry participants and academic institutions are all driving market growth.
Based on type, the market is classified into bacterial consortia transplantation (BCT)/fecal microbiota transplantation (FMT), peptides, live biotherapeutic products, and other types. In 2022, the bacterial consortia transplantation (BCT)/fecal microbiota transplantation (FMT) category dominated the global market. The rising incidence of illnesses linked to the microbiome, such as obesity, inflammatory bowel disease (IBD), and Clostridium difficile infection (CDI), is blamed for this increase.
Faecal matter from a healthy donor is transferred to a recipient patient during both BCT and FMT treatments. On the other hand, BCT makes use of a specific group of bacteria that have been demonstrated to be advantageous to human health. FMT, on the other hand, makes use of a donor’s entire faecal sample.
Segmentation:
Human Microbiome Market, By Product |
|
Human Microbiome Market, by Type |
|
Human Microbiome Market, by Disease |
|
Human Microbiome Market, By Geography |
|
Frequently Asked Questions (FAQs):
- Which key factors will influence human microbiome market growth over 2023-2030?
The main reasons anticipated to propel the human microbiome market during the forecast period are the expanding number of R&D activities, the ageing population base, the prevalence of lifestyle-related disorders, and the rise in personal disposable income.
- What will be the value of the human microbiome market during 2022-2030?
According to the report, the global human microbiome market size was worth USD 560.57 million in 2022 and is estimated to grow to USD 3,256.65 million by 2030, with a compound annual growth rate (CAGR) of approximately 24.60% over the forecast period.
- Which region will contribute notably towards the human microbiome market value?
North America is anticipated to continue leading the human microbiome market throughout the projected period.
- Which are the major players leveraging the human microbiome market growth?
Some of the main competitors dominating the global human microbiome market include – Seres Therapeutics, Inc. (US), Enterome (France), 4D pharma plc (UK), International Flavors & Fragrances Inc. (US), OptiBiotix Health Plc (UK), Ferring Pharmaceuticals (Switzerland), Synlogic, Inc. (US), Second Genome, Inc. (US), Vedanta Biosciences, Inc. (US), YSOPIA Bioscience (France), FlightPath Biosciences, Inc. (US), Finch Therapeutics Group, Inc. (US), AOBiome Therapeutics (US), BioGaia (Sweden), Quantbiome, Inc. (dba Ombre) (US), Viome Life Sciences, Inc. (US), BIOHM Health (US), DayTwo (US), Atlas Biomed (UK), Bione Ventures Private Limited (India), Luxia Scientific (France), Metabiomics (US), Sun Genomics (US), Seed Health (US), and Gnubiotics Sciences (Switzerland).
-
GET FREE DATA ACCESS!